
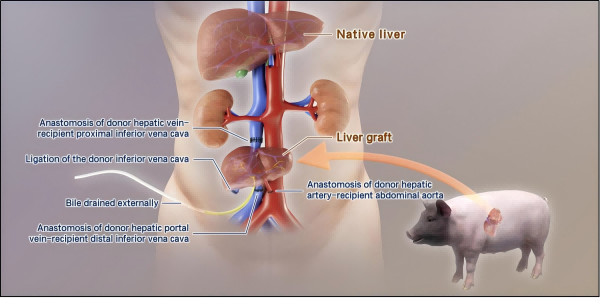
Schematic diagram of pig human xenograft liver transplantation
With the support of National Natural Science Foundation of China projects (approval numbers: 82325007, 82371793, 82070671, 82170667), the teams of Professor Dou Kefeng, Professor Wang Lin, and Professor Dong Hailong from Xijing Hospital of the Fourth Military Medical University have made progress in xenotransplantation. The research, titled "Gene edited pig to human liver xenotransplantation," was published online in the journal Nature on March 26, 2025. The link to the paper is: https://www.nature.com/articles/s41586-025-08799-1 .
Worldwide, patients with end-stage liver disease face a severe shortage of donor organs for survival. As an innovative solution to address organ shortages, gene edited pig xenotransplantation technology has made new progress. Following successful human transplantation of hearts and kidneys, Chinese research teams have achieved important research results in the field of xenotransplantation of liver.
This study used six gene edited pigs (silenced pig derived immune rejection genes and expressed human derived immune tolerance genes) as the donor liver source, and transplanted them to brain dead patients. The patients were observed and monitored for ten days after surgery. Prior to transplantation, it was confirmed that GGTA1, B4GALNT2, and CMAH genes, which cause hyperacute rejection in peripheral blood mononuclear cells and liver tissue of donor pigs, have been inactivated. Human complement regulatory proteins CD46/CD55 and antithrombotic protein TBM are significantly overexpressed. Accurate matching of vessel size during ectopic assisted liver transplantation involves connecting the hepatic vein and portal vein of the donor liver to the inferior vena cava system of the recipient, anastomosing the hepatic artery of the donor liver with the abdominal aorta, and establishing bile drainage. Postoperative ultrasound showed that the hepatic artery blood flow velocity remained stable at 41.45~60.63 cm/s, and the portal vein blood flow parameters were normal. Immediately after transplantation, bile secretion appeared in the donor liver, and the level of porcine albumin significantly increased; Until the observation endpoint, although there was a brief increase in alanine aminotransferase, core indicators such as alanine aminotransferase and alkaline phosphatase remained normal, and coagulation function remained generally stable and fluctuated within the physiological range. Complement activation, cellular and humoral immune responses are effectively inhibited. No cross species transmission of porcine endogenous retroviruses and cytomegalovirus was found in the study. This study indicates that by combining the six gene editing strategy of donor pigs with the specific immunosuppressive regimen of recipients, the hyperacute and acute rejection reactions of xenotransplantation can be successfully controlled, allowing pig derived liver to exert certain physiological functional substitution abilities in the human body (Figure).
This study demonstrates important achievements in the field of xenotransplantation, laying the foundation for the clinical application of xenotransplantation and providing new intervention ideas for the treatment of acute liver failure or the life maintenance of patients waiting for allogeneic liver transplantation.