
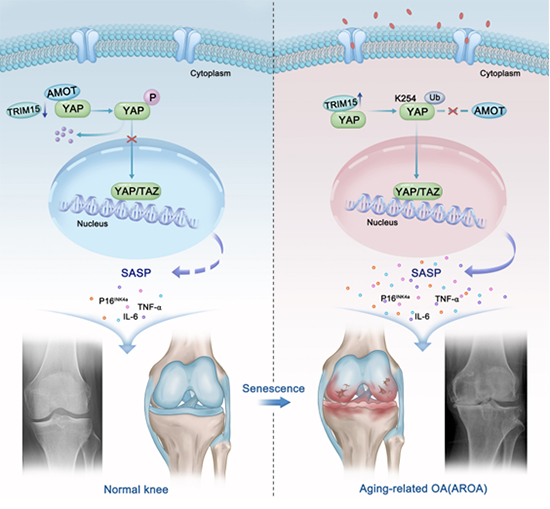
Molecular mechanism of TRIM15 regulating the microenvironment of cartilage aging
With the support of National Natural Science Foundation projects (approval numbers: 82322045, 81972105, 82272557) and other grants, the team of Associate Chief Physician Ji Mingliang and Army Chief Physician from Southeast University Affiliated Zhongda Hospital, in collaboration with the team of Researcher Shen Shuying from Run Run Shaw Hospital affiliated with Zhejiang University School of Medicine, has made progress in the research of cell aging promoting the formation of osteoarthritis. The research findings, titled "TRIM15 drives chondrocyte senescence and osteoarthritis progression," were published on March 26, 2025 in the journal Science Translational Medicine. Paper link: https://www.science.org/doi/10.1126/scitranslmed.adq1735 .
Osteoarthritis is a common chronic joint disease characterized by degenerative changes in articular cartilage, secondary bone hyperplasia, and inflammatory changes around the joints. These pathological changes often lead to joint pain, stiffness, and functional impairment, with a very high disability rate in the late stage, seriously affecting the quality of life of patients. Cellular senescence refers to the gradual decline in proliferation and differentiation ability, as well as physiological function, of cells over time during the execution of life activities. It has been confirmed to be associated with pathological changes in various diseases, but its mechanism of action in promoting the progression of osteoarthritis has not been elucidated.
The research team conducted RNA seq analysis on the diseased tissues of patients with osteoarthritis and found that genes containing triple motif containing protein 15 (Trim15) were upregulated in the diseased tissues and enriched in cell aging related pathways; After further validation through a population queue, phenotype identification was performed on the conditionally knocked out mouse strain, and it was found that knocking out Trim15 can significantly inhibit chondrocyte aging and the progression of osteoarthritis. Molecular mechanism studies have found that TRIM15 protein with increased expression binds to YAP protein, mediating the modification of its K254 site (human origin) by K48 ubiquitin chain, leading to enhanced YAP nuclear translocation, driving the expression of cell aging related genes, and promoting chondrocyte aging. In vivo animal experiments and ex vivo human tissue experiments have found that adenovirus 5 loaded Trim15 shRNA can alleviate chondrocyte aging and osteoarthritis progression (Figure).
This study reveals the molecular mechanism of targeting TRIM15 to alleviate chondrocyte aging and inhibit the progression of osteoarthritis by regulating the YAP signaling pathway, providing a new strategy for the prevention and treatment of osteoarthritis.