
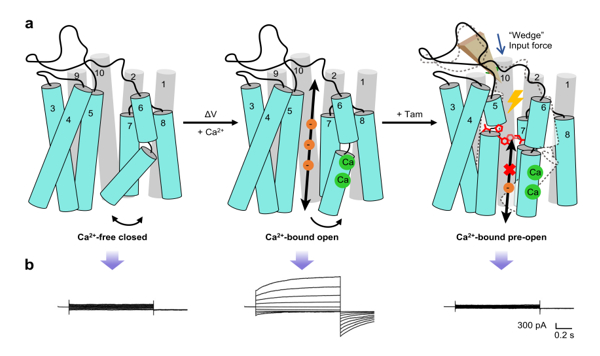
Schematic diagram of the conformational gating and regulatory mechanism of
Tamsulosin combined with TMEM16A extracellular domain hidden pocket
With the support of the National Natural Science Foundation of China
projects (approval numbers: 82192880, 82192882) and other grants, the research
teams led by Li Yingxian from the China Astronaut Research and Training Center,
Professor Li Honglin from East China Normal University, and Ling Shukuan from
the Oujiang Laboratory have made progress in the study of new uses of old drugs
and their mechanisms of action. The research findings, titled "Tamsulosin
Amelirates bone loss by inhibiting the release of Cl ⁻ through wedging into a
novel allosteric site of TMEM16A", were published online on December 31, 2024 in
the Proceedings of the National Academy of Sciences. Paper link:
https://www.pnas.org/doi/10.1073/pnas.2407493121 .
With the acceleration of global population aging, osteoporosis is becoming
an increasingly severe public health problem. Transmembrane protein 16A
(TMEM16A) is a calcium activated chloride ion channel, but due to insufficient
understanding of its regulatory mechanism, the development of effective
inhibitors still faces significant challenges.
The research team used the self-developed molecular three-dimensional
similarity method SHAFTS (SHApe FeaTrue Similarity, a mixed computing method
that simultaneously considers molecular shape feature similarity) and found that
the drug Tamsulosin, which is used to treat benign prostatic hyperplasia,
exhibits good inhibitory activity against TMEM16A (IC50=7.22 μ M). Subsequently,
the complex structure of TMEM16A and Tamsulosin was analyzed using cryo electron
microscopy technology (Protein Database (PDB) ID: Using 8XLR with a resolution
of 2.93 Å, it was found that Tamsulosin wedged into a novel implicit pocket of
the extracellular domain of TMEM16A, stabilizing the pre opening transient
conformation of TMEM16A under Ca2+activation, preventing pore opening and Cl -
permeation. The key roles of arginine 605, glutamic acid 624, tyrosine 593, and
isoleucine 641 in regulating Tamsulosin binding and pore conformation and
activity were further validated through the comprehensive application of
molecular dynamics simulation, electrophysiology, point mutation, and functional
experiments. Tamsulosin effectively inhibits TMEM16A current, suppresses
osteoclast differentiation, reduces the expression of key osteoclast marker
genes, and inhibits bone resorption by regulating Cl - concentration and
signaling pathways such as Syk Btk PLC γ 2 and CaMKIV-CREB-NFATc1. In a mouse
model of osteoporosis induced by ovariectomy, Tamsulosin significantly improved
bone density and structure, reduced bone resorption marker CTX-1, and osteoclast
gene expression (Figure).
This study discovered a new use of the old drug Tamsulosin through
computational simulation combined with pharmacology, and confirmed through
structural biology methods that Tamsulosin wedges into the target TMEM16A,
squeezes out hidden conformational sites. At the same time, it verified the
feasibility of protein transient conformation as a drug development target,
providing new research ideas and inspirations for drug targets and original drug
discovery.