
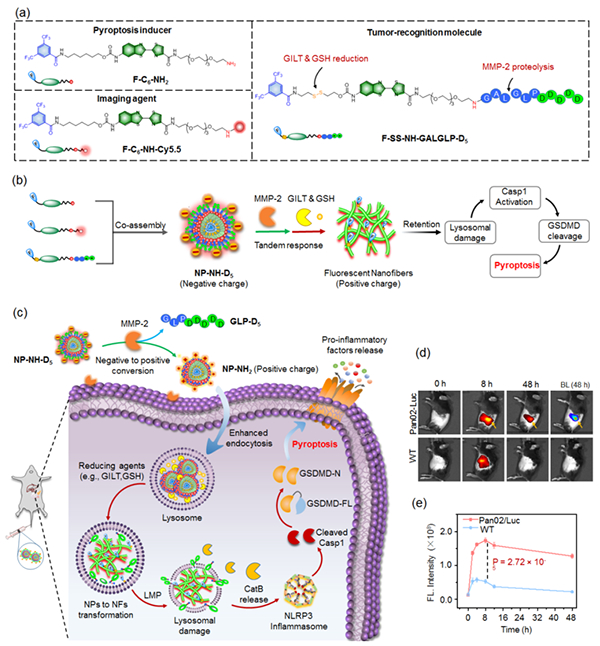
Figure (a-c) Schematic diagram of molecular assembly probes that can
cascade in response to the tumor microenvironment and their research;
Fluorescence imaging and signal intensity changes of in situ pancreatic cancer
in (d, e) mouse model
With the support of National Natural Science Foundation of China projects
(approval numbers: 22274074, 2137003) and other grants, the team led by Ye Deju
from Nanjing University has made new progress in the study of molecular probes
in vivo. The related research results, titled "Tandem controlled lysosomal
assembly of nanofibers induces pyroptosis for cancer immunotherapy", were
published on February 18, 2025 in the journal Nature Nanotechnology. Paper link:
https://doi.org/10.1038/s41565-025-01857-9 .
Molecular probes are key tools for precise detection and regulation of
biomolecules, widely used in fields such as disease diagnosis, target
validation, drug development, and molecular mechanism research. However, the
complex biological tissue barriers, highly dynamic environment, and the presence
of a large number of interfering substances in vivo limit the specificity of
probes and the efficiency of target tissue delivery, which poses great
difficulties for precise detection and regulation of biomolecules in the current
complex living environment.
In response to the above challenges, the team has developed a molecular
assembly probe (NP-NH-D5) that can cascade in response to the tumor
microenvironment through modular design of molecules and precise regulation of
intermolecular assembly, combined with surface charge flipping and structural
morphology transformation strategies. This probe can sequentially recognize
extracellular MMP-2 enzyme and intracellular reducing biomolecules (GILT enzyme
and glutathione) in the tumor microenvironment, and activate them step by step
to regulate the surface charge, size, and structure of the probe, thereby
achieving efficient targeted delivery to tumor cells. This process effectively
enhances the uptake efficiency of the probe in the tumor and significantly
prolongs the retention time within the tumor, thereby enhancing the tumor
imaging signal and achieving precise localization of in situ and distal
metastatic tumor foci in the mouse model.
On this basis, the above team further explored the cascade process of
"targeting assembly destruction activation" of probes in tumor cells,
elucidating the mechanism by which in situ assembled nanofiber structures
rapidly destroy tumor cell lysosomes and activate GSDMD dependent cell
pyroptosis molecules. This provides an innovative chemical strategy for precise
localization and targeted immunotherapy of tumor lesions in complex living
environments.