
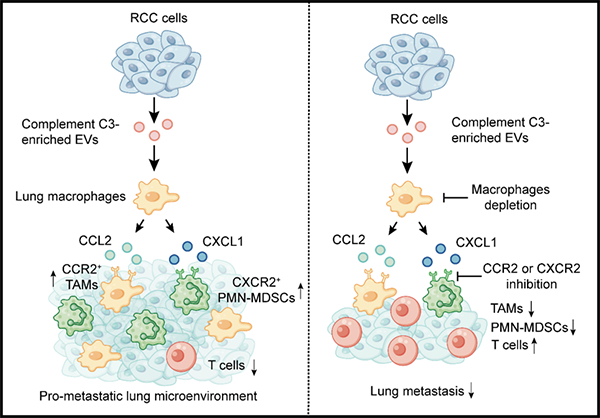
Functional pattern diagram of extracellular vesicle C3 promoting metastasis and recruiting immunosuppressive bone marrow cells
With the support of National Natural Science Foundation projects (approval numbers: 82025029, 82150114, 82430094), Professor Gao Shan's research group at Southeast University has made progress in the study of tumor cell-derived extracellular vesicles promoting metastasis. The research findings, titled "Complex C3 of Tumor derived Extracellular Vesicles Promotes Metastasis of RCC via Recruitment of Immunosuppressive Myeloid Cells," were published online on January 23, 2025 in the Proceedings of the National Academy of Sciences of the United States of America. The link to the paper is: https://www.pnas.org/doi/10.1073/pnas.2420005122 .
C3 is the central effector molecule of the complement system, which is activated through classical, lectin, or bypass pathways to produce active fragments C3a and C3b. These fragments not only participate in immune responses, but also play important roles in tumor development and progression. The C3a/C3aR axis promotes tumor metastasis by disrupting tight junctions between epithelial cells, activating tumor associated fibroblasts, and recruiting neutrophils. However, the mechanisms by which the complement system affects tumor progression and immune microenvironment remain largely unknown for exploration. The factors secreted by tumor cells, especially extracellular vesicles (EVs), play an important role in the formation of ecological niches before metastasis. The formation process includes vascular leakage, hematopoiesis, extracellular matrix remodeling, and the generation of immunosuppressive microenvironment. At present, the specific functions and mechanisms of EV C3 in tumor metastasis and pre metastatic niche formation are not yet clear.
This study used quantitative mass spectrometry analysis to compare the abundance of EV proteins secreted by renal cell carcinoma metastatic cells and in situ cells, and found that the content of complement C3 was elevated in EVs of metastatic cell lines. In terms of function, C3 does not affect metastasis in immunodeficient mice, but promotes metastasis in immunocompetent mice, indicating that the pro metastatic effect of C3 in vivo depends on the immune system. On a molecular level, EV C3 is absorbed by macrophages at the site of lung metastasis, promoting the secretion of chemokines CCL2 and CXCL1, which are responsible for recruiting tumor associated macrophages and multi nucleated bone marrow-derived immunosuppressive cells, respectively. After blocking the CCL2/CCR2 and CXCL1/CXCR2 axes with small molecule inhibitors, the recruitment and metastasis promoting effects of C3 were inhibited. In clinical practice, the overall survival and progression free survival of renal cell carcinoma patients with high expression of C3 are shorter than those with low expression of C3 (Figure).
This work reveals the molecular mechanism of the key molecule C3 in promoting metastasis of EVs derived from renal cell carcinoma, providing new targets and theoretical support for immunotherapy of renal cell carcinoma metastasis.