
With the support of National Natural Science Foundation projects (approval
numbers: 81930030, 82230036) and other grants, Professor Ma Huan's team at
Zhejiang University has made progress in the study of the neural plasticity
mechanism of brain dynamic "charging and energy supply". The research results,
titled "Boosting neural activity driven mitochondrial DNA transcription coupling
improves cognition in aged mice", were published in the journal Science on
December 20, 2024. The link to the paper is:
https://www.science.org/doi/10.1126/science.adp6547 .
As the core organ of dominant thinking and consciousness, the high
efficiency and low consumption of the brain is the goal that artificial
intelligence technology strives to imitate, and it is also the peak that human
technology has not yet reached. Meanwhile, the regulation of brain energy is
closely related to human health, and its imbalance is considered a key risk
factor for neurological diseases. Whether it is the energy shortage caused by
high energy consumption in the development of artificial intelligence or the
severe challenges brought by an aging society, they are major challenges that
humanity must face for survival and development today. From a scientific
perspective, understanding how the brains of mammals integrate the fundamental
elements of the life universe - energy matter information - not only provides
direction for mimicking or even surpassing the brain's ability to achieve low
consumption and high efficiency over the long course of evolution, but also
provides an important opportunity to explore solutions to aging related
problems.
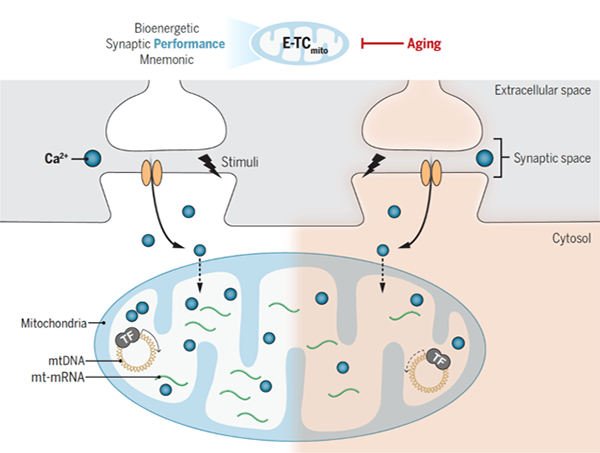
The research team utilized molecular biology, electrophysiology, and
behavioral techniques to discover that there is an age-related coupling between
neural activity and mitochondrial DNA (mtDNA) transcription, which differs from
the classical neural activity gene transcription coupling in the nucleus
(E-TCnuc). E-TMico utilizes molecules traditionally associated with E-TCnuc to
regulate mtDNA expression in regions closely related to synaptic activation. In
both in vitro and in vivo models, blocking E-TMito impairs activity driven mtDNA
expression and severely damages neuronal energy reserves, reducing the ability
to meet synaptic transmission needs. Further research has found that elderly
mice exhibit activity dependent reductions in mitochondrial calcium signaling
and mtDNA expression, suggesting age-related decline in E-TMito. In elderly
mice, the sustained activation of mitochondrial cyclic adenosine monophosphate
effector binding protein can restore activity dependent mtDNA expression,
increase neuronal energy reserve, and enhance memory function (Figure).
This study discovered a novel coupling mechanism in which the neural
activity of the brain during information processing can drive mitochondrial gene
transcription, and elucidated the neural plasticity mechanism of the brain's
dynamic "charging and energy supply". This achievement provides a new direction
for understanding the "energy-saving" and anti-aging effects of information
processing.