
In the National Natural Science Foundation of China project (Approval No.
81920108005) U23A20417、 With funding from organizations such as 81730007,
31872842, and 91442106, Professor Hong Dengli's research team at Shanghai Jiao
Tong University School of Medicine has made progress in revealing the mechanisms
by which embryonic tissues maintain stem cell genome stability and the origin of
diseases. The research results, titled "Fetal hepatocytes protect the HSPC
genome via fetuin-A through fetal globulin A", were published in the journal
Nature on December 4, 2024. Paper link:
https://doi.org/10.1038/s41586-024-08307-x .
During embryonic development, the extensive proliferation and
differentiation of the three germ layer cells is a key process in the formation
of diverse functional cell types and tissue organs. Maintaining the stability of
the tissue stem cell genome during this process is a crucial challenge. It is
currently unclear how developing organ tissues form a microenvironment and
transmit signals, thereby activating the endogenous genomic stability mechanism
of stem cells. In addition, it is urgent to study how the loss of this
protective mechanism can lead to the occurrence of developmental related
diseases, such as childhood tumors.
The research team established a liver cell tracking and knockout mouse
model and found that during the early development of fetal liver (the main
hematopoietic tissue of the fetus), a large number of hematopoietic stem and
progenitor cells (HSPCs) migrate from the placenta to the fetal liver. Due to
the small number of liver cells, the newly migrated HSPCs do not receive
effective microenvironment protection, resulting in genomic instability; When
stimulated by genotoxic substances, DNA breakage and mutation are prone to
occur, initiating the development of leukemia. As the fetal liver develops, the
number of liver cells increases and the genomic stability of HSPCs is
enhanced.
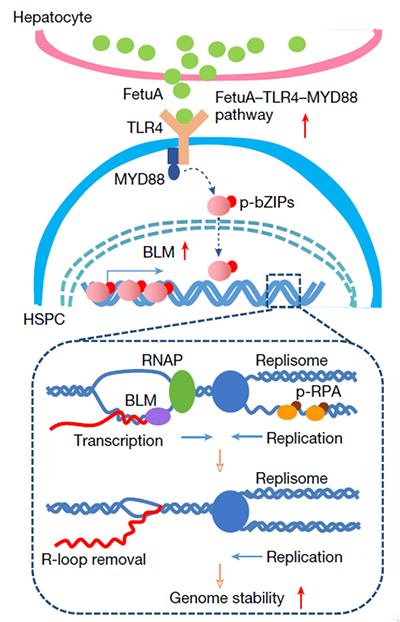
Further research has found that fetuin-A secreted by liver cells binds to
Toll like receptor 4 (TLR4) receptors on the surface of HSPCs, activating the
TLR4-MYD88-bZIP pathway and upregulating the expression of nucleic acid helicase
BLM, thereby releasing the R-loops structure generated by DNA replication
transcription in HSPCs and maintaining their genomic stability. If the Fetua
gene is knocked out in mice, the level of R-loops produced by the replication
and transcription of related genes is significantly increased, and the mice are
significantly susceptible to leukemia. The above protective mechanism has also
been validated in human fetal liver, and low concentrations of fetuin-A are
closely related to the occurrence of childhood leukemia.
The study revealed the key mechanism by which fetal liver cells maintain
the genomic stability of hematopoietic stem and progenitor cells. The research
results not only provide theoretical support and experimental evidence for
further studying the pathogenesis and prevention strategies of developmental
diseases, but also provide a scientific basis for the development of diagnostic
tools and therapeutic drugs for predicting and preventing childhood
leukemia.