
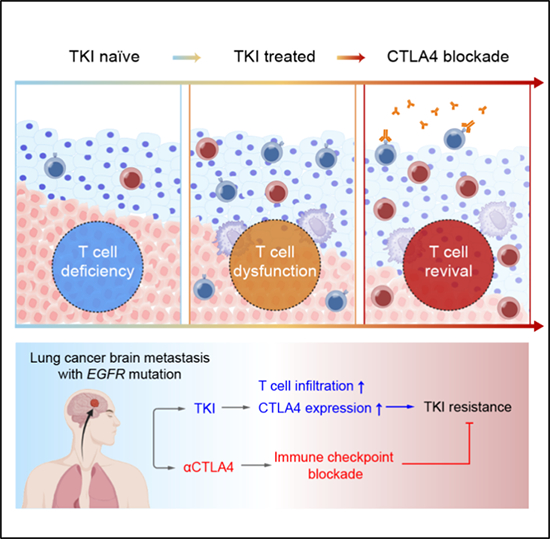
Targeting CTLA4 can revive anti-tumor immunity in lung cancer brain metastases that progress after targeted therapy
With the support of National Natural Science Foundation of China projects (approval numbers: 82072784, 82072785, 82273304, 82303437), the research team led by Professor Mao Ying, Professor Hua Wei, and Researcher Chi Yudan from Huashan Hospital affiliated with Fudan University has made progress in the study of immune escape involved in targeted therapy resistance for lung cancer brain metastasis. The research findings, titled "Overcoming tyrosine kinase inhibitor resistance in lung cancer brain metastasis with CTLA4 blockade," were published online on October 17, 2024 in the journal Cancer Cell. Paper link https://doi.org/10.1016/j.ccell.2024.09.012 .
Lung cancer is the leading cause of cancer-related deaths worldwide, and lung cancer patients with brain metastases have a worse prognosis. These patients urgently need new treatment strategies. In recent years, third-generation tyrosine kinase inhibitors (TKIs) have shown good efficacy in targeting EGFR mutations in patients with lung cancer brain metastases. However, the emergence of drug resistance significantly affects the sustainability and effectiveness of targeted therapy, and the combination of immunotherapy to improve targeted therapy resistance has become a current research focus.
The research team collected 31 samples of lung cancer brain metastases from patients with common gene mutations (such as EGFR, ALK, and TP53) and TKI targeted therapy. Using single-cell sequencing technology, a panoramic cellular microenvironment map of lung cancer brain metastases carrying different gene mutations was constructed. The results showed that compared to other types of metastatic tumors, the number of T cell infiltration in EGFR mutant lung cancer brain metastasis specimens was relatively small. Further analysis shows that TKI therapy can promote the release of HMGB1 from tumor cells, thereby increasing the degree of T cell infiltration. Meanwhile, infiltrating T cells can also directly recognize HMGB1 signaling, leading to upregulation of CTLA4 expression. The study also established a mouse model of lung cancer brain metastasis with EGFR mutation, and the combined treatment strategy of TKI and CTLA4 monoclonal antibody significantly reduced the tumor burden of mice and significantly prolonged their survival. It is encouraging that this combination therapy can effectively activate the function of T cells, revitalize anti-tumor immunity, and overcome immune escape after TKI treatment for lung cancer brain metastasis, providing a new perspective for the clinical treatment of lung cancer brain metastasis (Figure).
This study provides a detailed characterization of the relationship between the immune microenvironment of lung cancer brain metastases and targeted therapy, revealing the important role of TKIs in reshaping the immune microenvironment and elucidating the upregulation mechanism of CTLA4. By combining targeted CTLA4, anti-tumor immune restart is achieved, providing new ideas for the clinical treatment of target resistant lung cancer brain metastases patients.