
For researchers, the worst thing is that the experimental results have an expected trend but do not reach statistical significance. Especially in the field of clinical research, research results also need to have clinical significance. If the final conclusion is negative, then everything is in vain.
But this article is different. Traditional Chinese medicine research+negative results, debuffs piled up, and even a willow leaf can be sent!
Research on Traditional Chinese Medicine Ascends to the Top with the Lancet
On November 13th, Professor Lily Song/Craig Anderson's team from Fudan University, together with Professor Jianwen Guo's team from Guangdong Provincial Hospital of Traditional Chinese Medicine, published an article titled "FYTF-919, a multicenter, randomized, placebo-controlled, double-blind clinical trial," in the top medical journal Lancet (IF=98.4).
This study was conducted in 26 hospitals across 12 provinces in China, involving a total of 1648 patients with acute cerebral hemorrhage. The safety and efficacy of FYTF-919 were reliably evaluated in patients with moderate to severe acute cerebral hemorrhage.

It is worth mentioning that this is the first time in The Lancet's history that a multicenter clinical study focusing on traditional Chinese medicine has been published. Although the result is regrettable. This large-scale randomized placebo-controlled double-blind clinical trial showed that the traditional Chinese medicine compound FYTF-919 did not demonstrate efficacy in functional recovery, survival, and health-related quality of life in patients with moderate to severe cerebral hemorrhage.
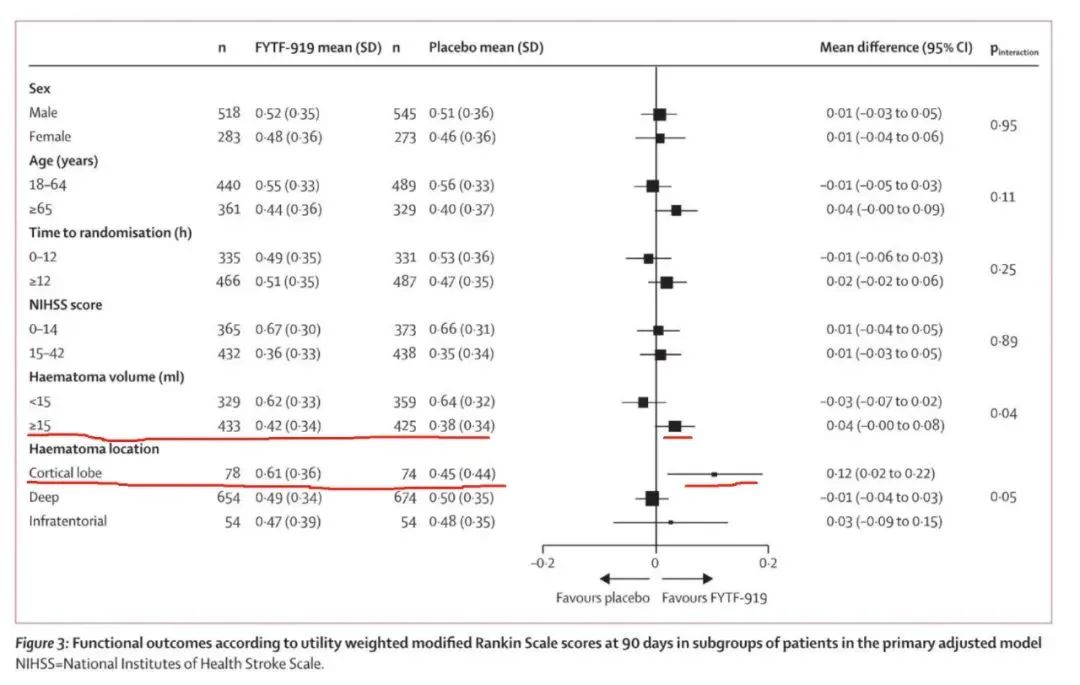
However, in the subgroup analysis, statistical differences were found in the treatment effect in terms of bleeding volume and bleeding location, indicating potential benefit signals in the patient population with cerebral hemorrhage exceeding 15ml and lobar hemorrhage. The subgroup results can only be used as exploratory conclusions, and the actual efficacy needs further verification.
Finally, the author describes it as a 'neutral result' in the text.
Two top publications were published in a day, both with negative results!
On November 20th, Professor Mao Ying's team from Huashan Hospital in Shanghai collaborated with Professor Liu Jianmin's team from Changhai Hospital to publish a research paper titled "Middle Meningeal Artery Metabolism for Non acute Subdural Hematoma" in the top medical journal NEJM (IF=96.2).
The study aims to evaluate the effectiveness and safety of auxiliary middle meningeal artery embolization in the treatment of non acute subdural hematoma. It was conducted under the guidance of the Development Center of Shanghai Shenkang Hospital, the National Center for Neurological Diseases (Huashan), and the Institute of Cerebrovascular Disease of the whole army (Changhai), led by Professor Mao Ying's team from Huashan Hospital affiliated with Fudan University and Professor Liu Jianmin's team from the First Affiliated Hospital of Naval Medical University, in collaboration with 31 neurosurgery and cerebrovascular disease centers in China.
The research team included 727 symptomatic non acute patients with subdural hematoma accompanied by space occupying effects who met the criteria. According to the clinical judgment of the surgeon, the patient is assigned to receive drilling drainage or non-surgical treatment. Subsequently, each group of patients was randomly assigned in a 1:1 ratio to receive middle meningeal artery embolization (using liquid embolization material) or routine care.
The primary endpoint is the recurrence or progression of symptoms of subdural hematoma within 90 days after randomization. The secondary endpoints include clinical and imaging outcomes. The main safety endpoint is any serious adverse events (including death).

The final study confirmed that the combination of auxiliary middle meningeal artery embolization and traditional treatment did not show statistically significant differences in reducing hematoma recurrence or progression compared to traditional treatment alone.
On the same day, a team led by Liu Jianmin and Yang Pengfei from Shanghai Changhai Hospital published an article titled "Balloon guided catheter for endovascular thrombectomy in Chinese patients with acute ischemic stroke due to large vessel occlusion (PROTECT-MT): a multicenter, open label, blinded endpoint, randomized controlled trial" in The Lancet.
This experiment evaluated the effectiveness and safety of using a balloon guided catheter during endovascular thrombectomy compared to using a conventional guided catheter in patients with acute ischemic stroke caused by anterior circulation large vessel occlusion.
From February 7th to November 13th, 2023, the research team included 1698 patients for eligibility assessment, of which 329 patients were randomly assigned (164 to the balloon catheter group and 165 to the traditional catheter group). Due to safety concerns, the experiment was suspended and terminated on April 18, 2024.
The final results indicate that compared with traditional guiding catheters, using balloon guiding catheters for endovascular thrombectomy of intracranial large vessel occlusion can lead to poor functional recovery in patients. Due to the limitations of the experiment, more research is needed in the future to confirm these results.
In fact, it is not uncommon for top journals to publish negative results as achievements. A reliable and informative negative result is also a significant discovery in itself, which can provide guidance for future research directions.
These negative results often have the following characteristics: ① treatment measures that have received widespread attention; ② Using standard treatment as a control; ③ New drug research. At the same time, data collection, experimental process design, scientific statistical methods, and a skilled team are all indispensable. Among them, teamwork is of utmost importance, having big shots in charge makes publishing articles smoother.
Of course, the successive publication of this research by Chinese scholars also indicates that China's scientific research strength has been further recognized internationally.