
He has 8 Natrude articles, 2 Science articles, and 3 Cell articles

Li Xiaochun started studying at Tsinghua University in 2004, under the guidance of Academician Shi Yigong. During his studies at Tsinghua University, he demonstrated outstanding research and exploration abilities, focusing on the field of protein structure and function research.
After obtaining his doctoral degree in 2012, Li Xiaochun went to Rockefeller University in the United States and joined the laboratory of Professor G ü nter Blobel, the winner of the 1999 Nobel Prize in Physiology or Medicine. He conducted postdoctoral research and won the Junior Researcher Breakthrough Prize from three institutions (Memorial Sloan Kettering Cancer Research Center, Rockefeller University, and Weill Cornell Medicine).
In 2017, he joined the Department of Molecular Genetics at the University of Texas Southwestern Medical Center as an assistant professor, where he continued to use structural techniques to study pathways related to cholesterol metabolism and lysosome storage diseases under the leadership of Professor Joseph Goldstein and Professor Michael Brown (1985 Nobel laureates in Physiology or Medicine).
Up to now, Li Xiaochun's team has published more than 40 papers at this level, and this year's research results are still fruitful, with 4 papers published and a total impact factor of over 122! (You can add me on WeChat to get the full text~)
01
Cell (IF=45.5)
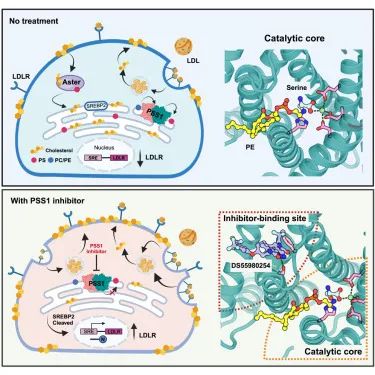
On October 3rd, Li Xiaochun, as the sole corresponding author, published a research paper titled "Molecular Insights into Human Phosphatidylserine Synthase 1 Reveal Its Inhibition Promotes LDL Uptake" in the top tier journal Cell. This study reveals the mechanism of mammalian PS synthesis and suggests that selective PSS1 inhibitors may lower blood cholesterol levels.

Title: Molecular insights of human phosphatidylserine synthase 1 reveal its inhibition promotes LDL uptake
Research content:
In mammalian cells, two types of phosphatidylserine (PS) synthases drive the synthesis of PS. Functional acquired mutations in the Ptdss1 gene lead to increased PS production, resulting in Lenz Majewski syndrome (LMS). Previously, pharmacological inhibition of PSS1 has been shown to suppress tumorigenesis.
This study reports for the first time the cryo electron microscopy structures of wild-type human PSS1 (PSS1WT), Pro269Ser mutant causing LMS (PSS1P269S), and PSS1WT complexed with inhibitor DS55980254. PSS1 contains 10 transmembrane helices (TMs), with TMs 4-8 forming the catalytic core located in the luminal lobules. These structures reveal that the working mechanism of PSS1 is similar to the speculated mechanism of the membrane-bound O-acyltransferase family. In addition, studies have found that both PS and DS55980254 can selectively inhibit PSS1, and the inhibitory effect of DS55980254 activates the SREBP pathway, thereby enhancing the expression of LDL receptors and increasing cellular uptake of LDL. This work reveals the mechanism of mammalian PS synthesis and suggests that selective PSS1 inhibitors may lower blood cholesterol levels.
02
Trends in Biochemical Sciences (IF=11.6)
On October 22nd, Li Xiaochun's research team published a review article titled "Clues into Wnt cell surface signaling and its biogenesis" in Trends in Biochemical Sciences,

Title: Wnt cell surface signal bodies and clues to their biological origins
Research content:
Wnt morphogens induce signal transduction by binding to their extracellular receptors. This article discusses several recent structural studies, demonstrating the interaction between Wnt and receptors frizzled (FZD) and low-density lipoprotein receptor associated protein 5/6 (LRP5/6), the role of Cachd1 as a substitute promoter for Wnt signaling, and how lipidated Wnt is produced and secreted within cells. These studies have enhanced our understanding of the Wnt signaling pathway and its regulatory mechanisms.
03
Nature communications (IF=14.7)
On May 23rd, Li Xiaochun, as the corresponding author, published an article titled "Structure and Inhibition of the Human Lysosomal Transport Sialin" in Nature Communications.

Title: Structure and inhibitory effects of human lysosome transporter sialic acid protein
Research content: This study presents four structural states of human Sialin using low-temperature electron microscopy technology: ligand free cytoplasmic open state, ligand free cavity open state, NAAG bound state, and inhibitor bound state. The structure shows that positively charged cytoplasmic open vestibules can accommodate NAAG or Sialin inhibitors Fmoc Leu OH, and their intracavity cavities may bind sialic acid. In addition, functional analysis and molecular dynamics simulations identified key residues that bind to sialic acid and NAAG.
In summary, this study analyzed the different structural states of Sialin and revealed how it simultaneously transports sialic acid and neurotransmitters through different mechanisms. This provides an important structural and functional basis for a deeper understanding of the function of lysosomal transport systems and the pathogenesis of related diseases, and for the development of drugs to treat lysosomal storage disorders.
04
Nature
On September 25th, the David Baker team at the University of Washington published an article titled "Designed endocytosis inducing protein degradation targets and amplification signals" in Nature, with Associate Professor Xiaochun Li as a co-author.

Title: Designed endocytosis induced protein degradation target and amplified signal
Research content:
This article introduces a new computational design method - EndoTags, which is used to overcome the limitations encountered by existing therapeutic methods in targeting protein degradation. EndoTags can fuse with target protein complexes, effectively guiding lysosomal transport and target degradation, especially suitable for receptors lacking natural ligands that can stimulate endocytosis. The study demonstrated EndoTags targeting multiple receptors, demonstrating their ability to achieve specific degradation in different tissues. In addition, EndoTags also demonstrate the potential to improve the signaling efficiency of engineered ligand receptor systems, with broad therapeutic application prospects.